Q2 2016 Update
Q2 2016 Update
Sydney, July 1, 2016 AEST (ABN Newswire) - Regeneus Ltd ( ASX:RGS) Q2 2016 Update and overview of activities.
ASX:RGS) Q2 2016 Update and overview of activities.
Advancing partnering opportunities in Japan
In late May, members of the executive team visited Japan to advance discussions with potential manufacturing and commercial partners for Progenza in Japan. We are pleased to report our meetings went well and we are on track to enter into our first significant partnering agreement in Japan by the end of September 2016. We look forward to updating the market on this transformational opportunity.
We are seeing increasing interest in Japan from large healthcare and technology companies for cellbased regenerative medicine technologies. This interest has grown since the introduction in late 2014 of new regulatory changes by Japan's Pharmaceuticals and Medical Devices Agency (PMDA) for an accelerated pathway to approval for regenerative therapies like Progenza.
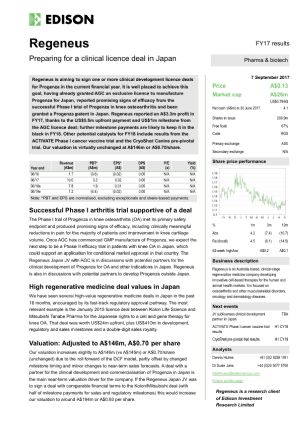
Progenza development pathway
In May, we reported that the Progenza Phase 1 STEP trial for knee osteoarthritis (OA) is fully recruited without any unexpected safety concerns to date. This outcome has helped in the progression of our partnering discussions in Japan. The study will complete once the last patient completes 12 months post treatment followup concluding with a second MRI.
We will move forward with the regulatory registration pathway in 2 jurisdictions. Our main aim is to progress towards GMP manufacture for a Phase 2 trial in Japan. This will include donor procurement, process development and technology transfer to our chosen GMP manufacturing facility. For the second jurisdiction (USA), we will complete some further preclinical studies (prior to submitting an Investigational New Drug (IND) application for a Phase 2 clinical study in the US). These studies will build upon the efficacy seen in our rabbit OA study. The results from the Australian Phase 1 STEP trial will also be helpful with the FDA pathway.
The rise of combination therapy of cancer vaccines and immune checkpoint inhibitors RGSH4K, our cancer vaccine, belongs to a group of products called immunotherapies. These types of products work to stimulate the immune system into recognising and attacking tumour cells. RGSH4K is a vaccine made from a patient's tumour cells and administered back to the same patient to act on the cancer.
We have now recruited patients in each of the three cohorts for the ACTIVATE Study. This Phase 1 dose escalating study is evaluating the safety, tolerability and preliminary efficacy of RGSH4K and will treat 21 patients with advanced cancers. We have opened a second tumour collection site and are seeing increasing tumour banking activity with a view to trial enrolment.
Cancer is a complex disease but immunotherapy is an exciting area of research showing very good benefit to patients, significantly increasing survival over standard therapies. A promising group of immunotherapy products is called checkpoint inhibitors, some of which are approved and available for patient use. Checkpoint inhibitors act to 'release the brakes' and stop the cancer from blocking the action of the body's immune cells.
Other therapies, such as cancer vaccines, work to 'step on the accelerator' to specifically focus the immune cells in a targeted attack against the cancer. Combining these types of therapies is giving even more beneficial results and is actively being tested in several cancer types.
ARC Grant to explore using stem cells for chronic pain
In May, we announced the Australian Research Council (ARC) had awarded a Linkage Grant of $340,000 to a research consortium that includes Regeneus collaborating with leading researchers from Macquarie University and the University of Adelaide to progress research into treating chronic pain with next generation stem cells.
The changing biopharma risk equation
The changing biopharma risk equation is an Economist Intelligence Unit (EIU) report sponsored by Merck. It draws on a multinational survey conducted in March 2016 of 254 pharmaceutical executives. The report found that pharmaceutical companies are in an expansive mode. With rapid advances being made in the development of new therapies, including stem cell derived therapies and gene therapies, and a growing cohort of potential customers in the burgeoning middle classes of emerging markets, expansion into both new product categories and geographic regions is a priority for most companies.
Australia in top 5 for global biotech for 3rd year running
Scientific American Worldview: A Global Biotechnology Perspective has launched its eighth annual scorecard at the BIO International Convention in San Francisco, ranking Australia in the top 5 for the 3rd year running. Australia also producted the 12th highest output on the Nature Index 2015 Global.
For further links and information, please visit:
http://abnnewswire.net/lnk/6TA30459
About Regeneus Ltd
 Regeneus Ltd (ASX:RGS) is a Sydney-based clinical-stage regenerative medicine company using stem cell technologies to develop a portfolio of novel cell-based therapies. The regenerative therapies seek to address unmet medical needs in human health markets, focusing on neuropathic pain, including osteoarthritis and various skin conditions, with its platform technologies Progenza(TM) and Sygenus. Visit www.regeneus.com.au for more information.
Regeneus Ltd (ASX:RGS) is a Sydney-based clinical-stage regenerative medicine company using stem cell technologies to develop a portfolio of novel cell-based therapies. The regenerative therapies seek to address unmet medical needs in human health markets, focusing on neuropathic pain, including osteoarthritis and various skin conditions, with its platform technologies Progenza(TM) and Sygenus. Visit www.regeneus.com.au for more information.





![abnnewswire.com]()
Related Companies
Social Media
Share this Article

 ASX:RGS) Q2 2016 Update and overview of activities.
ASX:RGS) Q2 2016 Update and overview of activities.  Regeneus Ltd (ASX:RGS) is a Sydney-based clinical-stage regenerative medicine company using stem cell technologies to develop a portfolio of novel cell-based therapies. The regenerative therapies seek to address unmet medical needs in human health markets, focusing on neuropathic pain, including osteoarthritis and various skin conditions, with its platform technologies Progenza(TM) and Sygenus. Visit www.regeneus.com.au for more information.
Regeneus Ltd (ASX:RGS) is a Sydney-based clinical-stage regenerative medicine company using stem cell technologies to develop a portfolio of novel cell-based therapies. The regenerative therapies seek to address unmet medical needs in human health markets, focusing on neuropathic pain, including osteoarthritis and various skin conditions, with its platform technologies Progenza(TM) and Sygenus. Visit www.regeneus.com.au for more information.